Consider the dissolution of CaCl2.
CaCl2(s) 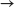 Ca2+(aq) + 2 Cl-(aq)
Ca2+(aq) + 2 Cl-(aq) 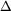 H = -81.5 kJ
H = -81.5 kJ
can someone help me with the equation and the solution?
Welcome to the Newschoolers forums! You may read the forums as a guest, however you must be a registered member to post. Register to become a member today!
CaCl2(s) 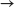 Ca2+(aq) + 2 Cl-(aq)
Ca2+(aq) + 2 Cl-(aq) 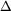 H = -81.5 kJ
H = -81.5 kJ